Short peptid catalysts in asymmetric organic synthesis
Employing quantum-chemical and density functional
based methods, we elucidate stereoselective effects
in organic catalysis. Short peptids are used to
catalyze C-C forming reactions, e.g. aldol addition,
Michael addition, allylation and many others.
Our theoretical investigations are carried out in close collaboration
with Prof. Svetlana Tsogoeva's experimental group at
the
The project has started in March 2005 and is part of the DFG Schwerpunktprogramm
Organokatalyse.
Among the numerous asymmetric carbon-carbon bond forming reactions, the
conjugate Michael addition plays a particularly important role. In particular,
the Michael reaction of ketones with nitroolefins represents a convenient access to g-nitroketones which are valuable building blocks in organic
synthesis.
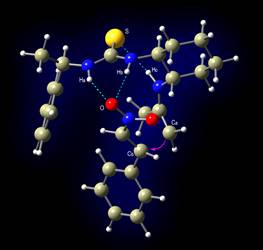
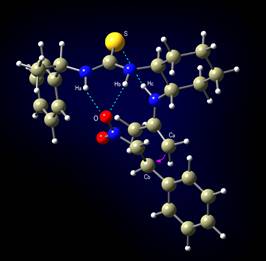
A new bifunctional organocatalyst, bearing both a thiourea
moiety and a primary amino group is used.
|
Transition state leading to R isomer |
Transition state leading to S isomer |
|
|
|
Recent papers:
New highly enantioselective
thiourea-based bifunctional
organocatalysts for nitro-Michael addition reactions,
S. Wei, D. A. Yalalov, S. B. Tsogoeva and S. Schmatz,
Catalysis Today 121, 151 (2007).
Chiral thiourea-based bifunctional
organocatalysts in the asymmetric nitro-Michael addition:
A joint experimental-theoretical study,
D. Yalalov, S. B. Tsogoeva and S. Schmatz,
Adv. Synth. Catal.
348,
826 (2006).