Ultrafast decomposition of organic
peroxides
The photochemical decomposition of large organic peroxides of general
structure RC(O)OOR' starts with optical excitation
into a repulsive electronic state. Within one vibrational
period, scission of the weak O-O bond occurs and a carbonyloxy
radical, RCO2, and an alkoxy radical,
R’O, are formed. Both intermediate species can undergo follow-up
processes. E.g., the carbonyloxy radical dissociates
into an aryl or alkyl radical R and carbon dioxide:
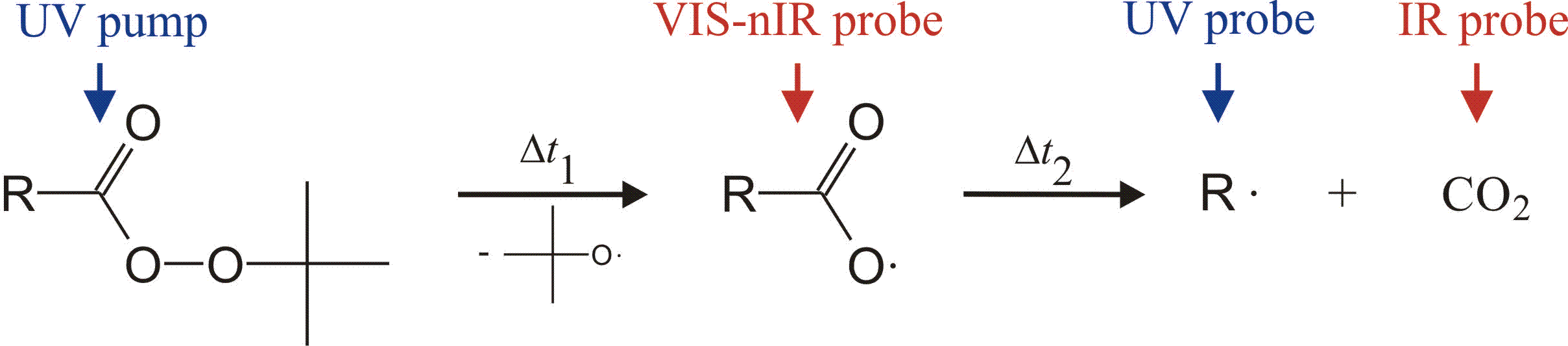
Large organic peroxides belong to a group of molecules of fundamental as
well as high practical importance. They are widely used as initiators in
free-radical polymerization. Investigations on initiator dissociation
mechanisms to answer the question of whether CO2 formation occurs
promptly or with some time delay are of eminent importance. The kinetics of
such processes determines the time evolution of different types of intermediate
radicals, carbon-centered or oxygen-centered, which in turn strongly affects the initiator
efficiency for free radical polymerization.
From different absorption spectroscopy experiments (UV-VIS, IR) with femtosecond time resolution we know the temporal evolution
of the intermediates and the formed products. The experiments are carried out
in the groups of Profs. Abel, Buback and Schroeder
at IPC Göttingen.
The aim of our research is to quantitatively understand the experimental
traces. A combination of quantum-chemical calculations, mainly at the DFT level
of theory, and statistical unimolecular
rate theory is employed for this purpose. The results are very encouraging and
allow for a prediction of the decomposition dynamics of other peroxides.
A missing reaction
step in dithiobenzoate-mediated RAFT polymerization,
M. Buback, O. Janssen, R.
Oswald, S. Schmatz and P. Vana,
Macromol. Symp. 248, 158 (2007).
Decomposition of tertiary alkoxy radicals,
M. Buback, M. Kling and S. Schmatz,
Z. Phys. Chem. 219, 1205 (2005) [special issue
in honor of Prof. Dr. E. U. Franck].
Photo-induced decomposition of
organic peroxides: Ultrafast formation and decarboxylation of carbonyloxy
radicals,
M. Buback, M. Kling, S. Schmatz and J. Schroeder,
Phys. Chem. Chem. Phys. 6, 5441 (2004) [invited article].
“Hot article”
Femtochemistry of organic peroxides: ultrafast formation and
decarboyxlation of carbonyloxy
radicals,
B. Abel, M. Buback, Ch.
Grimm, M. Kling, S. Schmatz and J. Schroeder,
in: C. Hynes and M. Martin (Eds.) Ultrafast molecular events in chemistry and biology,
Proceedings of the Femtochemistry VI, Elsevier, p.
287 (2004), ISBN 0444516565.
Ultrafast decarboxylation of carbonyloxy
radicals: Influence of molecular structure,
B. Abel, J. Assmann, M. Buback, C. Grimm, M. Kling, S. Schmatz,
J. Schroeder and T. Witte,
J. Phys. Chem. A 107, 3499 (2003).
A seemingly well understood
light-induced peroxide decarboxylation reaction
reinvestigated with femtosecond time resolution,
B. Abel, M. Buback, M.
Kling, S. Schmatz and J. Schroeder,
J. Am. Chem. Soc. 125, 13274 (2003).
Decarboxylation of carbonyloxy radicals: A density functional
study,
M. Kling and S. Schmatz,
Phys. Chem. Chem. Phys. 5 , 3891 (2003).
Experimental and theoretical investigations
of the ultrafast photoinduced
decomposition of organic peroxides in solution: Formation and decarboxylation of benzoyloxy
radicals,
B. Abel, J. Assmann, P. Botschwina, M. Buback, M. Kling,
R. Oswald, S. Schmatz, J. Schroeder and T. Witte,
J. Phys. Chem. A 107, 5157
(2003).
Ultrafast decarboxylation of organic peroxides in
solution: Interplay of different experimental approaches and theoretical
modelling,
B. Abel. J. Assmann, M. Buback, M. Kling, S. Schmatz and
J. Schroeder,
Angew. Chem. 115,
311 (2003);
Angew. Chem. Int. Ed. 42, 299 (2003).