
Kinetics of decomposition processes
The process of phase separation in the solid state is of great technical relevance not only for metallic systems in which precipitation hardening is frequently used to improve the performance of materials but also for soft matter like polymer blends which become more and more important in recent times. Hence, the mechanism of decomposition and the formation of topological structures due to the precipitation of a second phase has been studied extensively in the past using both, theoretical and experimental techniques. The extension of a miscibility gap in a two- or more-component system is directly related to the interaction between the constituents and can be described by thermodynamic quantities. More detailed information about system properties can, however, be obtained by investigating the kinetics of decomposition: The observation of transient states in a non-equilibrium system which is transforming from a homogeneous state into a two-phase state allows to characterise trajectories and time-evolution even on a microscopic scale.Several mechanisms are usually distinguished depending on the preparation of the demixing system. Nucleation and growth processes are believed to dominate the phase separation after a quench into the metastable region of the miscibility gap. Here, the free energy can be reduced by concentration fluctuations of large amplitudes but small spatial extension. If, however, a homogeneous system is cooled into the thermodynamic unstable region below the spinodal, fluctuations of small amplitudes and large wavelengths become stable and are able to grow as a function of time - a continuous process which is known as spinodal decomposition.
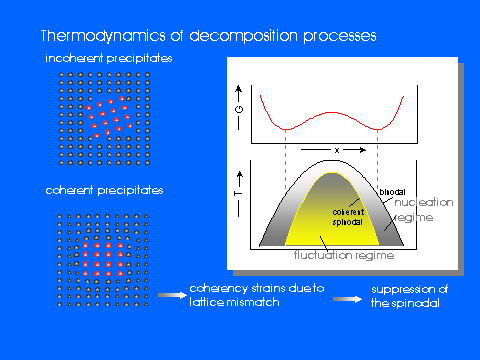
While a huge number of investigations on metallic or polymer systems are published, the kinetics of phase separation in ionic systems has hardly been studied. However, silver-, copper- or alkali halides with its rather large (cation) mobilities may be regarded as quite ideal model systems since the anion sublattice provides a more or less rigid frame for demixing processes which is almost completely confined to the cation subsystem.
In our group, the time-evolution of the demixing process in silver-alkali halides is studied by combining
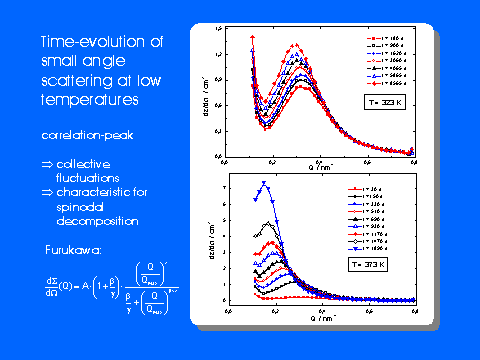
small angle neutron scattering (SANS) with time-resolved neutron diffraction and
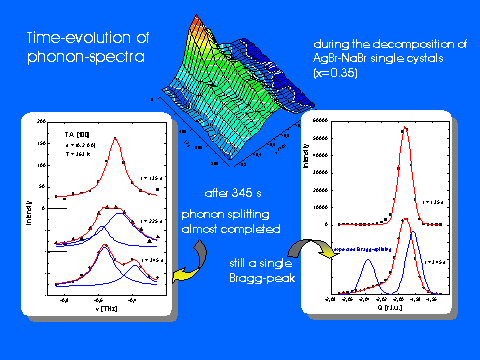
time-resolved inelastic scattering from phonons. While SANS provides information about the (time-dependent) size of precipitates or the characteristic wavelengths of concentration fluctuations, diffraction data reflect the relaxation of the anion lattice due to the different lattice parameters of the constituents. Most direct information about the local behaviour within the solid is obtained from phonon investigations since the sound velocities and also the optical phonon frequencies of the pure compounds differ appreciably. Using stroboscopic techniques, we are able to determine observe changes of phonon spectra on a time scale of seconds.
Once details of the kinetics are known, controlled spinodal decomposition can be used to produce well defined nanostructures by quenching. A direct visualisation of patterned surfaces in single crystals is possible by
Atomic Force Microscopy.
Some selected results obtained for the model system AgBr-NaBr are shown in the figures below.
See also Komitee Forschung mit Neutronen - Neues aus der Forschung Page 1, Page 2.

Institut für Physikalische Chemie Tammannstrasse 6
37077 Göttingen
| eMail: | geckold@gwdg.de |